We are interested in the evolution of organellar mechanics within the green lineage
What is organellar mechanics?
It’s an umbrella term that includes both the mechanical properties of organelles (e.g., their stiffness or elasticity) as well as their ability to sense and respond to mechanical forces (mechanosensing).
Why do we care about it?
It is becoming clear that mechanical properties and mechanosensing of organelles directly affect their morphology and function, as well as the function and development of whole cells and organisms. So, it is imperative to understand the mechanisms underlying these processes. This is a new and very exciting field of research.
How do cells and organelles sense mechanical force?
One of the most widespread mechanosensing mechanisms is the action of mechanosensitive (MS) ion channels, which can directly activate and release ions in response to membrane tension/force. We are currently focusing on PIEZO and MSL families of MS channels.
What organelles are we studying?
The current focus is on vacuoles and chloroplast, arguably the two defining organelles of plant cells. Vacuoles are by far the largest plant organelles with many vital functions, including ion and metabolite storage, maintenance of cellular osmotic potential and pH, and autophagy. And chloroplasts do not need an introduction. As sites of photosynthesis, they directly or indirectly supply food and energy for most of life on Earth. We are also open to projects involving other organelles, so let us know what your favorite organelle to study is.
What is the green lineage?
It is one of the three lineages that arose after the primary endosymbiosis of cyanobacteria 1.5 billion years ago. This large evolutionary branch includes both green algae, land plants, as well as several other groups (see on the right).
Why study the evolution of organellar mechanics?
Over evolutionary time, different lineages faced different mechanical challenges and evolved many creative solutions. So, only by studying multiple extant lineages, we can learn about the diversity of organellar mechanical properties and signaling and how they contribute to the diversity of organellar morphologies and functions. Furthermore, we can also gain insight into the fundamental mechanisms or general rules underlying cell mechanics.
What species do we work with?
We work with representatives from several major green lineages. Our primary model is moss Physcomitrium patens which is representative of a very large and old Bryophyte lineage. Physco is also a great research model, due to its easy cultivation, genetic manipulation, and imaging. We also work with Arabidopsis thaliana, one of the best-studied flowering plant model species as well as the green alga Chlamydomonas reinhardtii; our newest model system we are still learning about. In the future, we are interested in adding more model systems that represent other green lineages (e.g., Charophyte algae).
Physcomitrium patens
Arabidopsis thaliana
Chlamydomonas reinhardtii
Vacuolar Mechanics
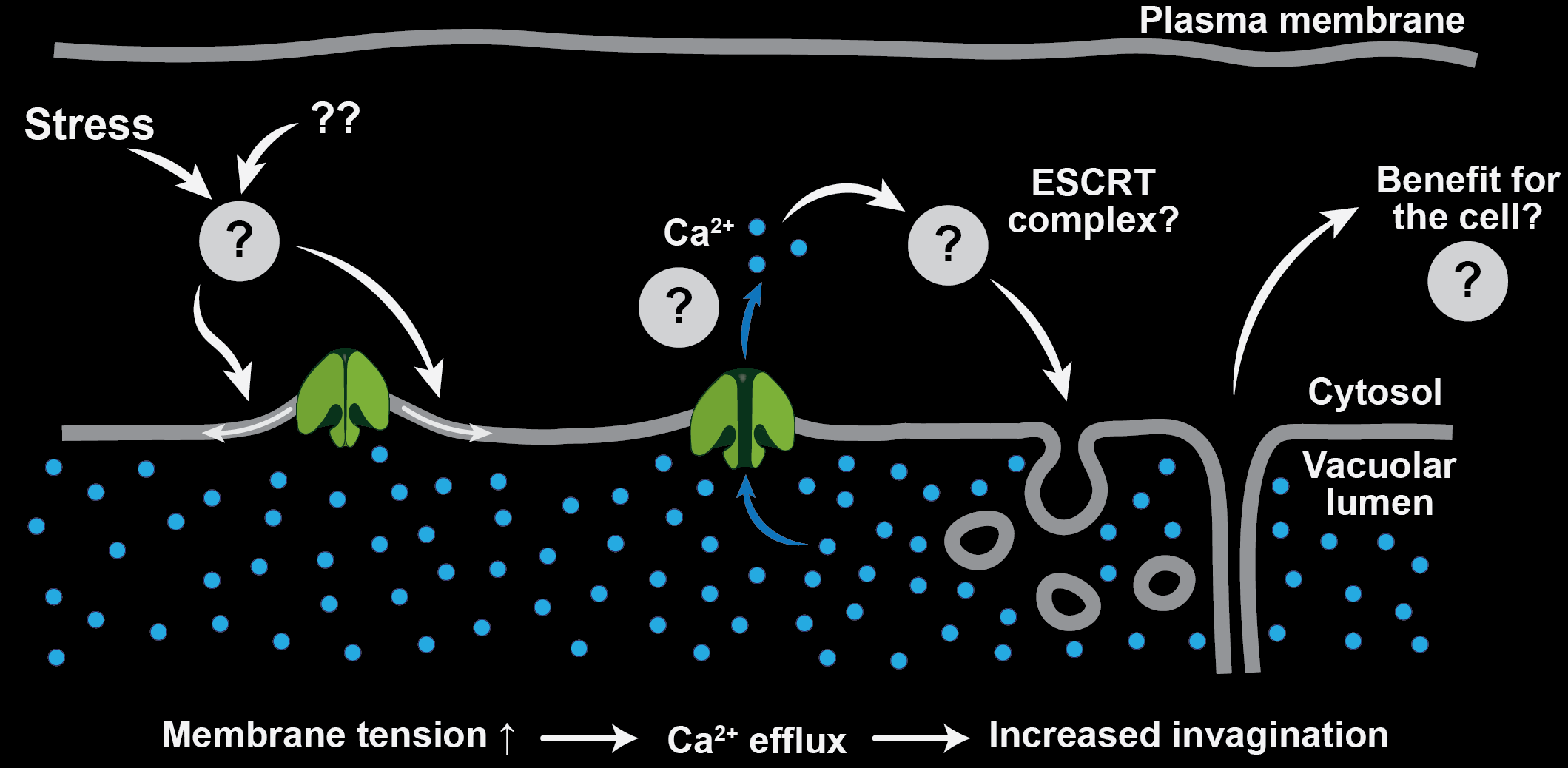
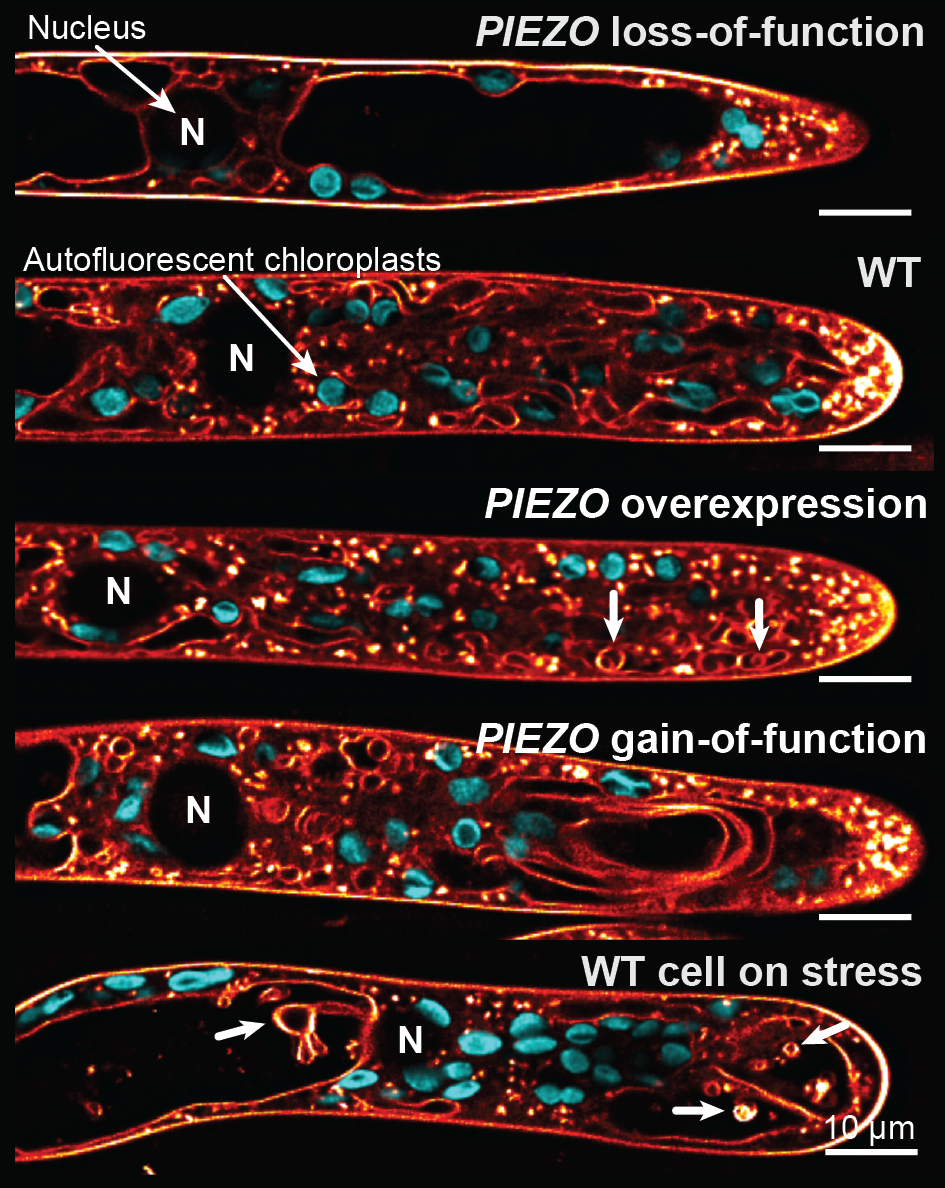
Recently we identified PIEZO channels as putative plant vacuolar mechanosensors. PIEZOs were first discovered in animals where these mechanosensitive calcium/cation channels play many essential functions including touch perception. However, land plant PIEZOs dramatically diverged from their animal counterparts both with respect to their subcellular localization and their function. Unlike plasma membrane-localized animal homologs, land plant PIEZOs localize to the vacuolar membrane and modulate vacuolar morphology in tip-growing cells.
One line of our current research is focusing on better understanding how PIEZOs function in the vacuolar membrane and how they promote membrane remodeling. As part of that effort, we are trying to determine when during the evolution were PIEZOs adapted for vacuolar function.
We are also interested in a big overarching question of why vacuoles need a mechanosensor. When do they experience mechanical forces inside the wall-protected cell? Some exciting preliminary data suggested that vacuolar mechanosensing plays a role in cellular stress perception and adaptation. We are following up on this.
The effect of PIEZO perturbation on vacuolar morphology in moss apical caulonemal cells
Chloroplastic Mechanics
Chloroplasts possess their own mechanosensitive ion channels, MSL2/3 from the MscS-like family. In Arabidopsis, these envelope-localized proteins control the osmotic homeostasis of chloroplasts, as evidenced by the dramatic swelling of plastids in msl2/3 double mutants (right panel in the figure). Physco has four MSL2 homologs, which appear to function differently than their Arabidopsis counterparts. This could be due to the presence of a thin bacteria-like peptidoglycan wall in the envelope of moss chloroplasts. Interestingly, the disruption of the PG wall in moss cells also leads to dramatic enlargement of chloroplasts (left panel in the figure).
We are interested in how the PG wall and MSL2 channels contribute to the mechanics and proper function of chloroplasts, and how have these functions changed over evolutionary time. Currently, we are testing the role of MSL2s in moss, their potential cooperation with PG, as well as the functional conservation of MSL2s across the green lineage.
If you want to learn more about our projects, please reach out to us. If you are interested in joining the lab to work on these and other projects, check out the current opportunities here.